
Angioedema Clinical Trial Pipeline Analysis Demonstrates 15+ Key Companies at the Horizon Expected to Transform the Treatment Paradigm, Assesses DelveInsight
Angioedema is a rapid swelling of the deeper layers of the skin or mucous membranes, often due to an allergic reaction or other triggers. Rising prevalence of allergic conditions and increasing awareness and diagnosis of hereditary angioedema (HAE) are key drivers fueling the growth of the angioedema treatment market.
/EIN News/ -- New York, USA, May 06, 2025 (GLOBE NEWSWIRE) -- Angioedema Clinical Trial Pipeline Analysis Demonstrates 15+ Key Companies at the Horizon Expected to Transform the Treatment Paradigm, Assesses DelveInsight
Angioedema is a rapid swelling of the deeper layers of the skin or mucous membranes, often due to an allergic reaction or other triggers. Rising prevalence of allergic conditions and increasing awareness and diagnosis of hereditary angioedema (HAE) are key drivers fueling the growth of the angioedema treatment market.
DelveInsight’s 'Angioedema Pipeline Insight 2025' report provides comprehensive global coverage of pipeline angioedema therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the angioedema pipeline domain.
Key Takeaways from the Angioedema Pipeline Report
- DelveInsight’s angioedema pipeline report depicts a robust space with 15+ active players working to develop 20+ pipeline angioedema drugs.
- Key angioedema companies such as Ionis Pharmaceuticals, Inc., Astria Therapeutics, Inc., Intellia Therapeutics, KalVista Pharmaceuticals, Ltd., Pharvaris Netherlands B.V., ADARx Pharmaceuticals, Inc., CAMP4 Therapeutics, and others are evaluating new angioedema drugs to improve the treatment landscape.
- Promising pipeline angioedema therapies such as Donidalorsen, STAR-0215, NTLA-2002, Sebetralstat (KVD900), Deucrictibant, ADX-324, Research programme: liver disorder therapeutic, and others are in different phases of angioedema clinical trials.
- In March 2025, A study was been published that stated that the treatments helping to control HAE boost life quality: Study in India Keeping symptoms of hereditary angioedema (HAE) under control is the most important factor contributing to a better quality of life, according to a study carried out by scientists reporting from India and noting severe limits on access to first-line medications there.
- In February 2025, Astria Therapeutics, Inc. announced the initiation of the ALPHA-ORBIT Phase III clinical trial of navenibart in people living with hereditary angioedema (HAE). Navenibart has the potential to provide rapid and sustained HAE attack prevention with a very low treatment burden and administration every 3 months (Q3M) and every 6 months (Q6M).
- In January 2025, Pharvaris, a biopharmaceutical company focused on developing treatments for bradykinin-mediated diseases, received orphan designation from the European Commission for its drug candidate deucrictibant, intended for bradykinin-mediated angioedema. This follows a similar designation by the U.S. Food and Drug Administration in March 2022.
- In January 2025, Intellia Therapeutics had dosed the first subject in a randomised, placebo-controlled global Phase III trial of its investigational CRISPR-based therapy, NTLA-2002, to treat hereditary angioedema (HAE). HAELO is a double-blind trial that will assess the therapy’s safety and efficacy in 60 adult subjects with Type I or Type II HAE.
- In January 2025, KalVista announced that sebetralstat was designated as an orphan drug (*1) by the MHLW, and a new drug application was submitted in January 2025. The NDA submission is supported by previously disclosed results, including data from the KONFIDENT Phase III clinical trial and ongoing KONFIDENT-S open-label extension trial.
Request a sample and discover the recent advances in Angioedema drugs @ Angioedema Pipeline Report
The angioedema pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage angioedema drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the angioedema clinical trial landscape.
Angioedema Overview
Angioedema is characterized by sudden, localized, non-pitting swelling of the deeper layers of the skin and mucous membranes, commonly affecting areas like the lips, face, neck, extremities, oral cavity, larynx, and gastrointestinal tract. It can become life-threatening when the larynx is involved, potentially obstructing the airway. When angioedema affects the intestines, it can cause severe abdominal pain and mimic signs of an acute abdomen. Hereditary angioedema (HAE), a rare form of the condition, is caused by genetic mutations in the C1-inhibitor gene and is inherited in an autosomal dominant pattern. Due to its rarity and varied presentation, especially when skin swelling is absent, HAE is often misdiagnosed or diagnosed late, particularly when there's no known family history or when symptoms are limited to gastrointestinal complaints.
Angioedema occurs due to increased permeability of local blood vessels, leading to fluid leakage into surrounding tissues. The release of histamine, bradykinin, or other chemical mediators typically drives this process. The most common type is histamine-mediated angioedema, often triggered by mast cell or basophil activation. When angioedema appears alongside hives, it usually indicates a histaminergic type, such as allergic or idiopathic histaminergic angioedema. However, the underlying cause depends on the specific kind of angioedema present.
Symptoms typically appear suddenly and may last up to three days. Common signs include swelling in areas like the hands, feet, genitals, face, tongue, throat, or intestinal lining. Other possible symptoms include a burning or tingling sensation, red itchy rashes, visual disturbances, abdominal pain, bladder issues, and difficulty breathing.
Diagnosis is primarily clinical, based on the timing, nature of symptoms, and potential triggers. A detailed history—including family background, recent medications, and possible allergen exposure—is crucial. If symptoms follow contact with a known allergen, allergic angioedema is suspected. A family history may suggest hereditary angioedema. Diagnostic tests may include skin prick testing for allergens, blood tests to assess immune reactions, and measurement of C1 esterase inhibitor levels and function—low levels or dysfunction indicate hereditary angioedema.
Treatment depends on the type and severity of angioedema. Many cases resolve without intervention, but avoiding known triggers is essential to prevent recurrence. In allergic or histaminergic cases, antihistamines, corticosteroids, or epinephrine (EpiPen) may be used. For hereditary angioedema, while there is no cure, treatment focuses on prevention and management using C1-inhibitor replacement therapies, fresh frozen plasma, or other targeted medications. In emergencies involving airway obstruction or breathing difficulty, immediate medical care is critical—securing the airway with a breathing tube may be necessary to ensure safety.
Find out more about angioedema drugs @ Angioedema Treatment
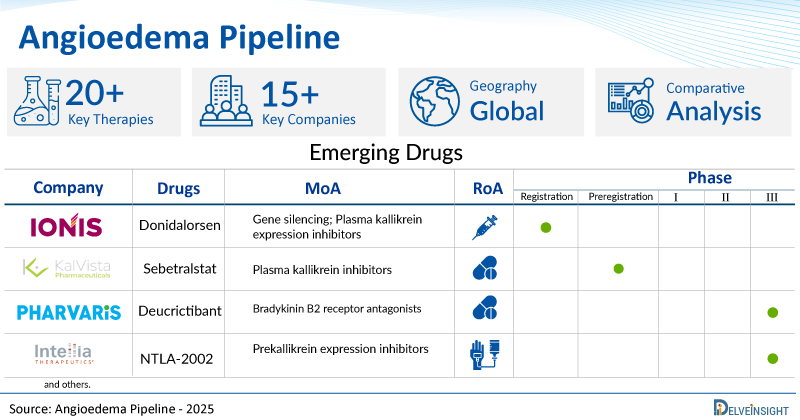
A snapshot of the Pipeline Angioedema Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| Donidalorsen | Ionis Pharmaceuticals | Registration | Gene silencing; Plasma kallikrein expression inhibitors | Subcutaneous |
| Sebetralstat (KVD900) | KalVista Pharmaceuticals | Preregistration | Plasma kallikrein inhibitors | Oral |
| Deucrictibant | Pharvaris | III | Bradykinin B2 receptor antagonists | Oral |
| NTLA-2002 | Intellia Therapeutics | III | Prekallikrein expression inhibitors | Intravenous |
| STAR-0215 | Astria Therapeutics, Inc | III | Plasma kallikrein inhibitors | Subcutaneous |
| ADX-324 | ADARx Pharmaceuticals | II | Prekallikrein expression inhibitors; RNA interference | Subcutaneous |
Learn more about the emerging angioedema therapies @ Angioedema Clinical Trials
Angioedema Therapeutics Assessment
The angioedema pipeline report proffers an integral view of the emerging angioedema therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Angioedema Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
- Therapeutics Assessment By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy
- Therapeutics Assessment By Mechanism of Action: Gene silencing, Plasma kallikrein expression inhibitors, Prekallikrein expression inhibitors, Bradykinin B2 receptor antagonists, RNA interference, Factor XIIa inhibitors,
- Key Angioedema Companies: Ionis Pharmaceuticals, Inc., Astria Therapeutics, Inc., Intellia Therapeutics, KalVista Pharmaceuticals, Ltd., Pharvaris Netherlands B.V., ADARx Pharmaceuticals, Inc., CAMP4 Therapeutics, and others.
- Key Angioedema Pipeline Therapies: Donidalorsen, STAR-0215, NTLA-2002, Sebetralstat (KVD900), Deucrictibant, ADX-324, Research programme: liver disorder therapeutic, and others.
Dive deep into rich insights for new angioedema treatments, visit @ Angioedema Drugs
Table of Contents
| 1. | Angioedema Pipeline Report Introduction |
| 2. | Angioedema Pipeline Report Executive Summary |
| 3. | Angioedema Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Angioedema Clinical Trial Therapeutics |
| 6. | Angioedema Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Angioedema Pipeline: Late-Stage Products (Phase III) |
| 8. | Angioedema Pipeline: Mid-Stage Products (Phase II) |
| 9. | Angioedema Pipeline: Early-Stage Products (Phase I) |
| 10. | Angioedema Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Angioedema Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Angioedema Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the angioedema pipeline therapeutics, reach out @ Angioedema Therapeutics
Related Reports
Angioedema Epidemiology Forecast
Angioedema Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted angioedema epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Angioedema Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key angioedema companies, including Ionis Pharmaceuticals, Inc., Astria Therapeutics, Inc., Intellia Therapeutics, BioMarin Pharmaceutical, Incyte Corporation, Novartis, KalVista Pharmaceuticals, Ltd., Sanofi, Pharvaris Netherlands B.V., ADARx Pharmaceuticals, Inc., CSL Behring, CAMP4 Therapeutics, among others.
Hereditary Angioedema Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key HAE companies, including Shire, Takeda Pharma, CSL Behring, Pharming Group, BioCryst Pharmaceuticals, Ionis Pharmaceuticals, KalVista Pharmaceuticals, among others.
Hereditary Angioedema Pipeline
Hereditary Angioedema Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key HAE companies, including BioCryst Pharmaceuticals, KalVista Pharmaceuticals, Pharvaris, BioMarin Pharmaceutical, Ionis Pharmaceuticals, Inc., Intellia Therapeutics, among others.
Hereditary Angioedema Epidemiology
Hereditary Angioedema Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted HAE epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679
www.delveinsight.com
Distribution channels: Banking, Finance & Investment Industry, Healthcare & Pharmaceuticals Industry, Media, Advertising & PR, Science ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release

